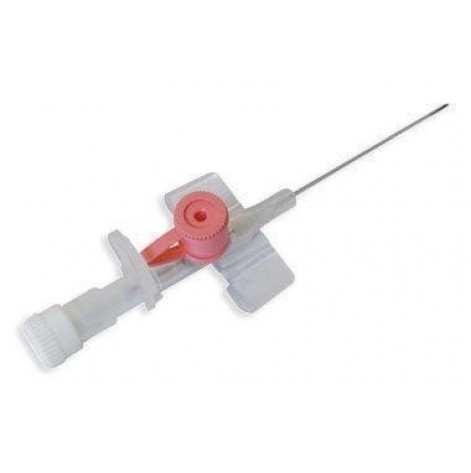
Most hospitalized patients receive fluids and medications through a peripheral venous catheter during some of their stay in the hospital.
A device that injects drugs into the human circulatory system (peripheral veins).
Specifications:
- The catheter tube is made of translucent polytetrafluoroethylene (PTFE, Teflon) or fluoroethylene propylene (FEP), materials with a very high level of biocompatibility. Catheters made of PTFE/FEP glide well and pose minimal risk for thrombosis;
- Needle cap - polypropylene. Needle cannula, reverse flow chamber and wings - polypropylene.
- The needle is made of austenitic medical steel with a trihedral sharpening of the needle;
- Side injection valve for additional intravenous injections;
- The injection valve is color-coded for size;
- Port with Luer slip or Luer Lock connection (Luer slip & luer lock);
- Wings are flexible for a clear fixation of the cannula;
- The presence of a hydrophobic filter in the backflow chamber of the cannula makes the cannula absolutely safe regarding the contact of medical personnel with the patient's blood containing hepatitis or AIDS viruses.
- Transparent backflow chamber to control the correct placement of the needle in the vein;
- A special plug protects against contact with the patient's blood;
- Radiopaque strip to control the position of the cannula in the blood vessel;
- Smooth transition between catheter and guide needle;
- Sterile, non-pyrogenic, non-toxic;
- For single use;
- Individual packing;
- Shelf life 5 years from the date of preparation indicated on the package.
Instructions for use:
- Select the desired size of the IV cannula. Try to use the smallest size cannula to administer the necessary intravenous fluids.
- Disinfect the puncture site of the blood vessel.
- Make sure that the blister pack of the cannula is intact, and that the product has not expired.
- Remove the protective cap of the needle and insert the needle at an acute angle into the peripheral vein.
- Verify that blood is flowing into the backflow chamber. This confirms the correctness of the puncture.
- Insert the catheter into the vein and at the same time carefully remove the needle.
- Do not attempt to re-insert a partially or fully withdrawn needle.
- Do not press your finger on the vein at or above the catheter insertion site. This may result in blood splatter.
- Connect the cannula to the system for the introduction of infusion solutions, a syringe or close the Luer lock plug.
- Dispose of the needle in an appropriate container.
- Secure the cannula with a patch.
- In an intravenous cannula with a port, drugs can be injected directly through the port using a syringe.
- If the port is not in use, it must be covered with a cap.
- Regularly check the vein site for any reactions.
- At the end of the manipulation, dispose of the cannula in accordance with established standards.